Some Manufacturers May Benefit from Advanced Planning and Scheduling (APS) in More Ways Than They Realize
- On 08/27/2019
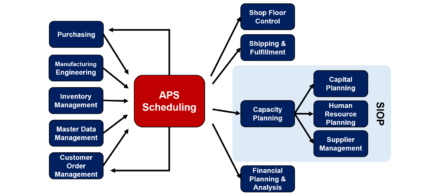
There are a variety of downstream business processes where APS can play a productive key role Advanced Planning and Scheduling (APS) is well known for its role in driving material purchasing and shop floor manufacturing operations. However, there are also a number of other business functions where APS can provide essential information to customers […]
Read More